Muscle Protein Loss in Aging and Cancer
Treatments
Management
Contact Us
Muscle Protein Loss in Aging and Cancer
Treatments
Management
Contact Us
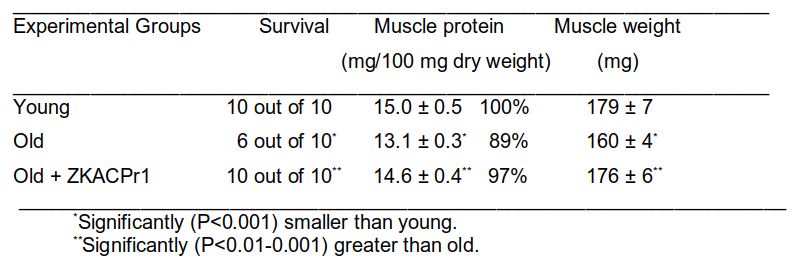
TABLE 1 shows the data from a typical experiment in which old mice were treated with a human protein, ZKACPr1, to increase survival of old mice as well as reduce the loss of muscle proteins and muscle mass.
Three groups of C57BL/6 mice were used. In the first group, 8 mice were kept on control diet for 6 months. In the second group (10 mice), mice were kept on control diet for 22 months. In the third group (10 mice), mice were kept on control diet and treated with ZKACPr1 once per week for the last 5 months (5 mg per kg body weight; subcutaneous application). M. gastrocnemius was isolated to measure muscle mass and determine total protein. The results, shown in TABLE 1, strongly suggest that in old people ZKACPr1 may be used to reduce reductions in muscle proteins and muscle weight and that these effects will translate into increased life expectancy.
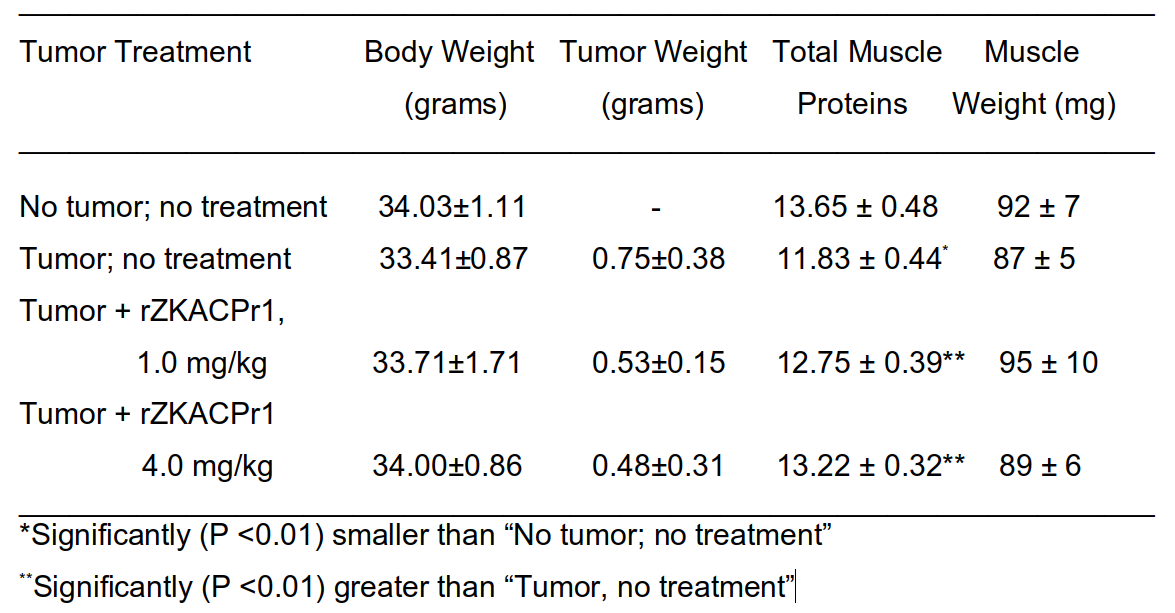
The next typical experiment performed with a non-cachectic tumor model to demonstrate that the loss of muscle proteins starts at early stages of tumor development when no changes in body weight and muscle mass occur as yet, and that this can be significantly reduced by recombinant ZKACPr1 (rZKACPr1).
In this experiment, tumor cells were implanted into 6 weeks old immune deficient male mice. The reason these mice were chosen because the transplanted tumors grow slowly that allowed separation of changes in muscle proteins, muscle mass/weight, and body weight.
Four groups of mice with 7 mice included in each group were used. The first group was tumor free control. In groups 2-4, human tumor cells were transplanted on day 0, when mice were 6 weeks old. Mice in Group 2 remained untreated, while mice in Groups 3 and 4 were treated with intravenous injection of 1.0 mg/kg and 4.0 mg/kg body weight of recombinant rZKACPr1 respectively, on days 11, 15, 18, 21, and 24 (days are counted from tumor transplantation). Total muscle proteins are expressed as: mg/100 mg of muscle weight.
Only measurements performed on day 25 in each group are shown in TABLE 2. No significant changes in the total body weight and muscle weight were recorded. This experiment established that in the time frame of the experiment, these tumor-bearing mice were not cachectic in the sense that they did not lose body weight and muscle weight. However, despite the relatively small tumor size and no apparent changes in body weight and muscle weight, tumor bearing untreated mice lost about 12% of skeletal muscle proteins compared to the “No tumor; no treatment” group.
rZKACPr1 at both doses reduced the loss of muscle proteins in a statistically significant manner. In other experiments, rZKACPr1 similarly reduced the loss of muscle proteins in chemotherapy (cyclophosphamide or cisplatin) treated tumor bearing cachectic mice.
These and similar experiments lead to the important conclusion that at the early stages of tumor development when tumors are still small, there is already significant loss of skeletal muscle proteins but not of body weight or muscle weight. This implies that if the treatment of cancer patients with rZKACPr1 starts right after tumor detection when tumors are still small, there is a greater chance to prevent development of wasting of muscle proteins (and perhaps also prevent the loss of body and muscle weight) compared to starting the treatment later when cachexia (significant loss of body weight along with substantial loss of skeletal muscle proteins and muscle mass) already irreversibly took hold. This also means that by starting adjuvant treatment with ZKACPr1 early, the efficacy of ongoing tumor treatments can be made more tolerable and more efficient.
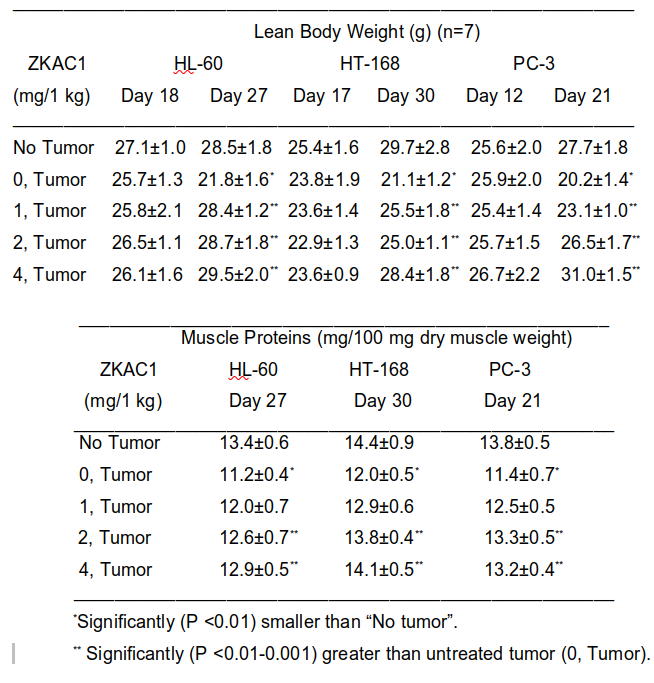
In the cachectic mouse tumor models (HL-60 human leukemia, HT-168 human melanoma, PC-3 human prostate cancer), a selected compound, ZKAC1 (selected from a family of similar compounds), greatly reduced both the loss of their body weight and muscle proteins (see TABLE 3 below) when daily subcutaneous treatment started soon after tumor transplantation and lasted until as shown, together with the results, in the TABLE. In each case, even these limited treatments with ZKAC1 increased survival by ~20-30% (not shown). ZKAC1 also has antitumor effects; in these models it inhibited tumor growth by 30-40%, and it also further increased the effects of antitumor agents (like cisplatin and cyclophosphamide) allowing the use of larger doses of them (data not shown).
Mechanism of action: Based on preliminary results, the company’s current hypothesis is that ZKAC1 inhibits the formation and/or transmission (via the cell membrane) of signals by the tumor involved in triggering cancer cachexia. Thus, ZKCA1 acts right at the source of cachectic signals.